Since 1995, Vivanco & García, has been providing translation services specializing in intellectual property, translations for industry and the chemical-pharmaceutical sector. We are always committed to meeting your needs regarding:
In Vivanco & García, our quality is accredited by ISO 9001:2015 and ISO 17100:2015 certifications (specific for technical translation services).
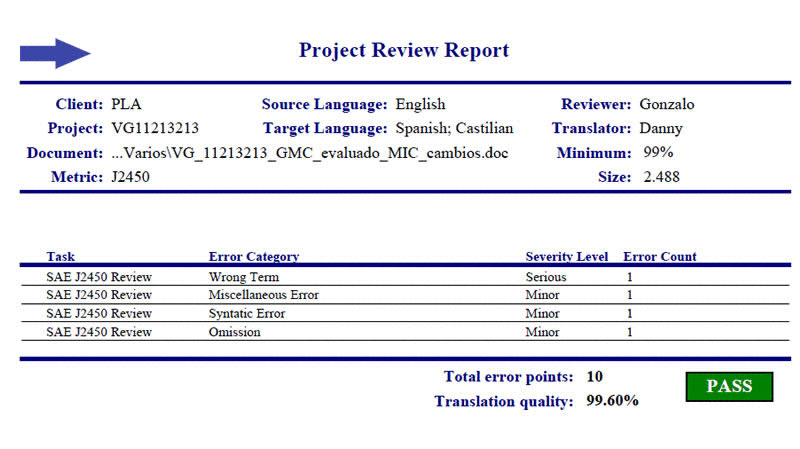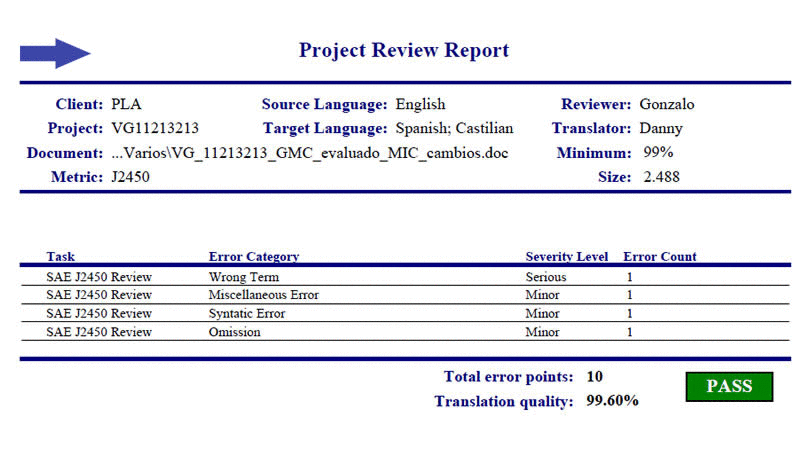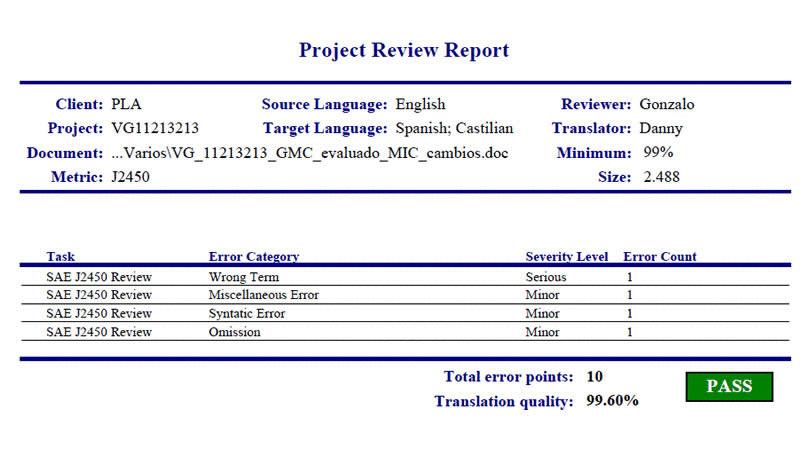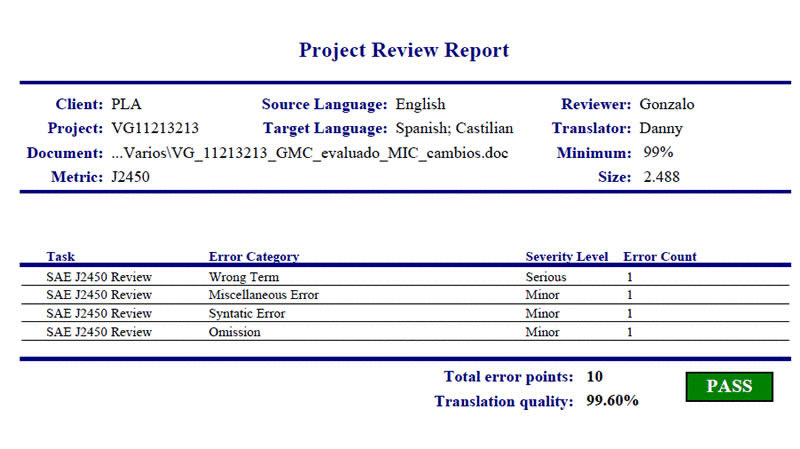
In 2007 we implemented a quality metric to assure the quality levels required in chemical-pharmaceutical and patent translation. Based on standard SAE J2450, this metric allows us to assure controlled quality through quality indices.
We work with IP agents in Europe and the Americas. We are also a provider for a large number of industrial and chemical-pharmaceutical companies from Germany, South America, Canada, United States, France, the Netherlands, Ireland, Israel, Italy, Japan, Portugal, United Kingdom, and Sweden after having passed demanding audits and quality tests.
Vivanco & García:

Since 1995, Vivanco & García has translated over 500 million of words in relation to industry, pharmaceutical laboratories, chemical laboratories, and intellectual property.
All documents are translated by our in-house staff who hold university degrees in engineering, chemistry, and biology. They are all selected and trained to meet your needs.
We promote the continued training of all our staff.
Our teams are trained to know, understand, and correctly apply the right terminology in the translation of patents and other specializations in German, Chinese, Spanish, French, English, and Portuguese in order to:
We have considerable consultation resources based on technical documentation, glossaries, and terminology integrated in modern technological tools.

Vivanco & García quality is designed to assure maximum peace of mind for our clients.
Our quality holds the following certifications:
Our certification of compliance with standard SAE J2450 gives us the opportunity to assure the highest quality levels for your translations of medical and pharmaceutical subjects, patents, and for industry.
All translation projects are subjected to strict controls integrated in our triple service and product quality system.
Our clients can rest assured knowing that:
And we guarantee that all projects are:

At Vivanco & García, we are convinced that peace of mind of our clients is based on achieving, maintaining, and improving our competitiveness and quality. For that reason, our quality is supported by the following principles:
This commitment to quality is understood, instilled, and maintained at all levels of our organization.

Confidentiality of the information sent to us is of vital importance for our clients.